Calorific Value: This term would be familiar to nearly all engineers or people working in petroleum sectors. But most of the folks don’t have an idea about it. So, if you are interested to have a brief idea about it you have peeped into a right page.
The fundamental definition which comes on googling it will convey something like this ” The energy contained in a fuel or food, determined by measuring the heat produced by the complete combustion of a specified quantity of it”.
But the rigorous study of its anatomy is not as facile as above interpreted. Aeroplanes, ship, helicopters, cars, don’t only have distinguished working or medium but also have distinguished fuels used in it. The frequent drivers might have observed that different petroleum companies have different rates of the same fuel in the same city. So what is the factor that decides these variations or categorizes the parent fuel crude oil into the vivid range of substitute fuels
And it is the calorific value that bifurcates a fuel into a different one by a little change in its digits. Fuels of all kind have an associate calorific value which can be determined as per the required use. Below is the list of calorific values of some fuel
Diesel |
10000 kcal/kg |
Natural gas |
12500 kcal/kg |
woods |
2500 kcal/kg |
Propane-butane |
11950 kcal/kg |
electricity |
860 kcal/kg |
Interpreting inversely, below table shows how much fuel to be burnt to produce 1kW energy:
Diesel |
0.085 kg |
Natural gas |
0.072 kg |
Propane butane |
0.073 kg |
charcoal |
0.124 kg |
gasoline |
0.083 kg |
This is just an overview of calorific values of familiar fuels. Apart from this, there are number of fuels which are categorically classified into its own kind. Eg. Kerosene itself has different types. The kerosene we use for general purpose is completely different for the one used in aeroplanes. I.e.unleaded kerosene or naphthalene blend.
How is the Calorific value determined?
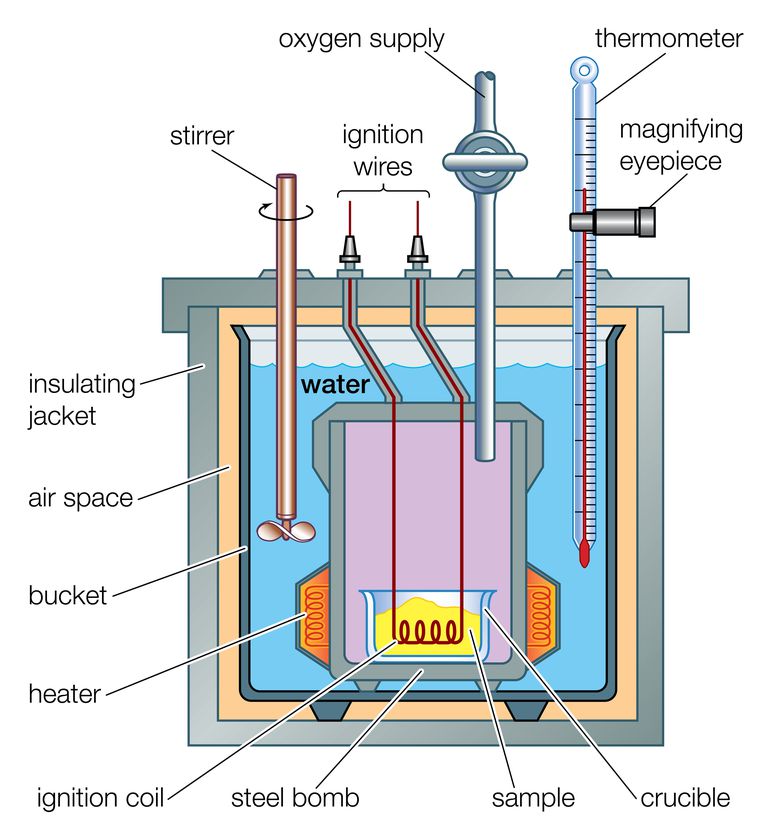 The instrument determining the calorific value of a fuel is known as Bomb Calorimeter. The procedure to carry out to calculate by this instrument is quite simple. The calorimeter is filled with a known mass of fuel and then the fuel is combusted. The initial and final readings are recorded by a thermometer which is changes due to the heating of surrounding water. And thus the calorific value is known by heat balance.
The instrument determining the calorific value of a fuel is known as Bomb Calorimeter. The procedure to carry out to calculate by this instrument is quite simple. The calorimeter is filled with a known mass of fuel and then the fuel is combusted. The initial and final readings are recorded by a thermometer which is changes due to the heating of surrounding water. And thus the calorific value is known by heat balance.






